Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is an ultra-rare acquired genetic disease characterized by uncontrolled complement activation, which results in intravascular hemolysis and an increased tendency to develop thrombosis. PNH causes symptoms such as severe fatigue, which can negatively impact a patient's physical functioning and health-related quality of life (QoL). Cemdisiran is an investigational N-acetylgalactosamine-conjugated small interfering RNA (siRNA) that suppresses liver production of complement component C5, while pozelimab is an investigational fully human monoclonal antibody inhibitor of C5. The combination of pozelimab and cemdisiran is being evaluated in an ongoing phase 2, randomized, open-label, two-arm study that is designed to evaluate the safety and efficacy of combination therapy in patients with PNH who were transitioning from pozelimab monotherapy (NCT04811716). Interim patient-reported outcomes data are presented here.
Methods: Twenty-two patients were randomized (1:1) to two treatment regimens; both arms received subcutaneous (SC) cemdisiran 200 mg every 4 weeks (Q4W) plus pozelimab 400 mg SC at a frequency of either Q4W (arm 1) or every 2 weeks (arm 2). Patients completed the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale (score range 0-52), and the European Organization for Research and Treatment of Cancer: Quality-of-Life Questionnaire global health status (GHS)/QoL and physical function assessments (score range 0-100). Higher scores indicate a better level of functioning or GHS/QoL, or less fatigue.
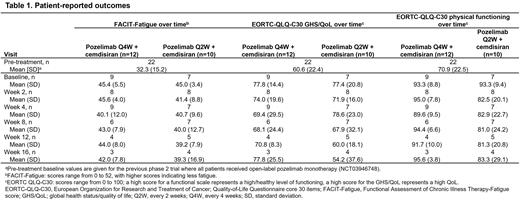
Results: Prior to receiving pozelimab monotherapy, mean (standard deviation [SD]) pre-treatment values were 32.3 (15.2) for FACIT-Fatigue scores, 60.6 (22.4) for GHS/QoL scores, and 70.9 (22.5) for physical functioning scores (Table 1). For the current trial, baseline values were representative of the pozelimab monotherapy that the patients were receiving prior to transitioning to the combination therapy. The mean (SD) FACIT-Fatigue score at baseline was 45.4 (5.5) for arm 1 and 45.0 (3.4) for arm 2 (Table 1). Over Weeks 2-16, the mean FACIT-Fatigue scores were 40.1-45.6 for arm 1 and 39.2-41.4 for arm 2. The mean (SD) GHS/QoL scores were well controlled and similar for both treatment arms at baseline (77.8 [14.4] for arm 1 and 77.4 [20.8] for arm 2). Over Weeks 2-16, the mean GHS/QoL scores were 68.1-77.8 for arm 1 and 54.2-78.6 for arm 2. The mean physical functioning score at baseline was 93.3 for both treatment groups (SD: 8.8 for arm 1 and 9.4 for arm 2). Over Weeks 2-16, the mean physical functioning scores were 89.6-95.6 for arm 1 and 81.0-83.3 for arm 2 (Table 1).
Conclusions: Patients with PNH who transitioned from pozelimab monotherapy had improved baseline scores compared with pre-treatment for their GHS/QoL, physical functioning, and fatigue scores. Improvements in these scores were maintained by the combination treatment through to Week 16, particularly with the pozelimab Q4W and cemdisiran dose regimen. This preliminary evidence, with limited sample size, suggests that pozelimab and cemdisiran combination therapy, particularly the Q4W regimen, maintains improvements in patient fatigue, GHS/QoL, and physical functioning.
Disclosures
Hartford:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Dain:Regeneron Pharmaceuticals, Inc.: Current Employment. Sherman:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Zhang:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Pavani:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Aurand:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Rofail:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Kelly:Swedish Orphan Biovitrum AB: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape; Biocryst: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference support; Sobi: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Biologix: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.